RESEARCH
Nanobiotechnology
1. Biological applications of Quantum dots
Semiconductor QDs present a possible new technology for in vivo bio-imaging and future medical-imaging applications. QDs have proven potential as imaging contrast agents due to their bright luminescence, their resistance to photobleaching, and their tunable emission wavelengths. The main impediment to the use of QDs for biological applications is their biocompatibility. QDs that contain toxic elements such as Cd, Pb, Hg, or As have limited biological applications. High quality ‘non-toxic’ QDs have been synthesized (e.g., InP, ZnTe/ZnSe, CuInS2; AgInS2); they are free from potential leakage of toxic heavy-metal ions, and photoluminesce at wavelengths that can be selected by adjusting the size of the QD. The new methods of synthesizing ‘non-toxic’ QDs using various solvents and QD precursors have been investigated to obtain bright and photostable QDs that promise application to in vivo medical imaging.
2. Surface modification, functionalization and bioconjugation of QDs for biological applications
QDs are synthesized in inorganic solvent to obtain high quality, so their surfaces should be modified for bioimaging applications. The surface molecules for QDs should give good colloidal stabilities in aqueous solutions without aggregation, and should have appropriate sites to allow conjugation with biomolecules. To be suitable for applications in targeted imaging or in long-term in vivo imaging, QDs must be colloidally stable and must have minimal non-specific binding. QDs that have been modified to have zwitterionic surface molecules can maintain colloidal stability over broad ranges of pH and ionic strength, and to show minimal non-specific adsorptions (Fig. 1). Moreover, the zwitterionic QD surface ligands can also be used in conjunction with other functional groups; this trait allows simple conjugations for highly specific targeting while retaining the advantages of a zwitterionic QD surface (Fig. 2).
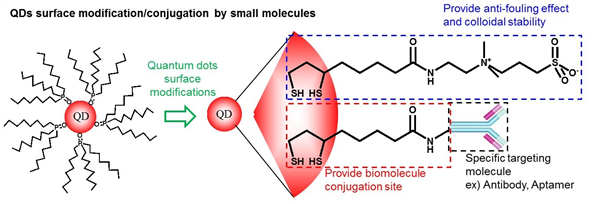 Figure 1. Scheme of surface modification of QDs which were synthesized in organic solvent. Zwitterionic QDs are colloidally very stable over a broad pH range and even in saturated NaCl solution, and they show minimal non-specific adsorptions. It can also be used in conjunction with other functional groups, which allows simple conjugations with minimal non-specific adsorption.
Figure 1. Scheme of surface modification of QDs which were synthesized in organic solvent. Zwitterionic QDs are colloidally very stable over a broad pH range and even in saturated NaCl solution, and they show minimal non-specific adsorptions. It can also be used in conjunction with other functional groups, which allows simple conjugations with minimal non-specific adsorption.
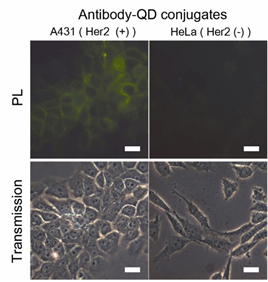 Figure 2. Fluorescence microscope images of A431 cells (HER2 positive) and HeLa cells (HER2 negative) after the treatment of Her2/neu-QD (QD) conjugates. A431 cells show fluorescence signal by specific targeting of antibody-QD conjugates whereas HeLa cells show low level of non-specific adsorption. (Adv. Funct. Mater .2011, 21, 1558-1566)
Figure 2. Fluorescence microscope images of A431 cells (HER2 positive) and HeLa cells (HER2 negative) after the treatment of Her2/neu-QD (QD) conjugates. A431 cells show fluorescence signal by specific targeting of antibody-QD conjugates whereas HeLa cells show low level of non-specific adsorption. (Adv. Funct. Mater .2011, 21, 1558-1566)
3. Cancer Diagnosis Using QD - Antibody Conjugates
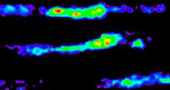
Cancer is a very heterogeneous system. Multiplexed QD imaging is well-suited to detecting such heterogeneous systems. QDs are also bright, photostable, and allow multiplexing by a single excitation wavelength. QDs have broad absorption profile with large one-photon and two-photon absorption cross sections, narrow and symmetric emission profile, and large effective Stokes shifts. To improve the accuracy of cancer detection, multiple biological targets must be imaged concurrently; these advantages of QDs can be used in multiplexing to achieve fluorescence molecular imaging of cancer tissues. Doctors frequently use endoscopy to detect colon cancer, but the diagnosis is based on the clinician's naked-eye perception, which is subjective and error-prone.We developed a rapid and accurate molecular fluorescence imaging technique that uses antibody (Ab)-coated QDs (Ab-QDs) that are sprayed and washed simultaneously on colon tumor tissues inside live animals, then excited and imaged by endoscopy. QDs were conjugated to matrix metalloproteinase (MMP) 9, MMP 14, or carcinoembryonic antigen (CEA) Abs with zwitterionic surface coating to reduce nonspecific bindings. The Ab-QD probes can diagnose tumors on sectioned mouse tissues, in fresh mouse colons stained ex vivo and in vivo, and in fresh human colon adenoma tissues in 30 min and can be imaged to a depth of 100 μm. The probes successfully detected not only cancers that are readily discernible to the naked eye, but also hyperplasia and adenoma regions. Sum and cross-signal operations provided post-processed images that can show complementary information or regions of high priority (Fig. 3). This multiplexed QD, spray-and-wash, and endoscopy approach provides a significant advantage for detecting small or flat tumors that may be missed by conventional endoscopic examinations and bestows a strategy for the improvement of cancer diagnosis.
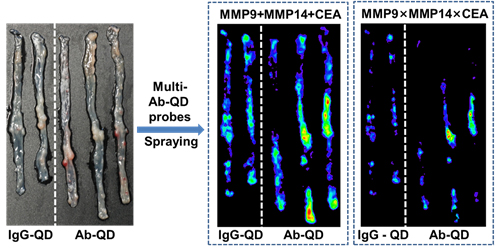 Figure 3. Multi-Ab-QD probes ex vivo staining images after the post-image processes by sum operation and cross operation for three QD signals.( ACS Nano , 2014, 8, 8896-8910)
Figure 3. Multi-Ab-QD probes ex vivo staining images after the post-image processes by sum operation and cross operation for three QD signals.( ACS Nano , 2014, 8, 8896-8910)
4. NIR-II emitting QD probes
The NIR biological window from 650 nm to 1700 nm allows deeper penetration than visible light into biological tissues such as skin and blood, because these tissues scatter and absorb less light at long wavelengths than at short wavelengths. The window between 650 nm and 950 nm (NIR-I) has been exploited extensively for in vivo optical imaging of live animals, but little research has considered use of the longer-wavelength window (NIR-II) from 1000 to 1700 nm for in vivo fluorescence imaging of live animals. Light scattering and absorption by biomolecules are much weaker in the NIR-II region than in NIR-I, so tissue penetration depth and spatial resolution are increased, and autofluorescence is decreased in NIR-II. QDs can be advantageous fluorescent probes for molecular imaging in both the NIR-I and the NIR-II region. CdTeSe, and CuInS2 have been developed as fluorophores in NIR-I, and have shown high photostability and quantum yield (QY) > 50%. PbS and Ag2S QDs have been developed for NIR-II fluorescence imaging applications, and showed QY > 10% with inorganic shells in the aqueous phase (Fig. 4). We report an enzyme-activatable NIR-II probe that exhibits fluorescence (FL) upon matrix metalloprotease activity in tumor microenvironment. Bright and stable PbS/CdS/ZnS core/shell/shell QDs were synthesized as a model NIR-II fluorophore, and activatable modulators were attached to exploit photoexcited electron transfer (PET) quenching. The quasi-type-II QD band alignment allowed rapid and effective FL modulations with a compact surface ligand modulator that contains methylene blue PET quencher. The modulator was optimized to afford full enzyme accessibility and high activation signal surge when the enzyme was active. In a colon-cancer mouse model, the probe demonstrated selective FL activation at tumor sites with tripled signal strength in 10 min (Fig. 5).
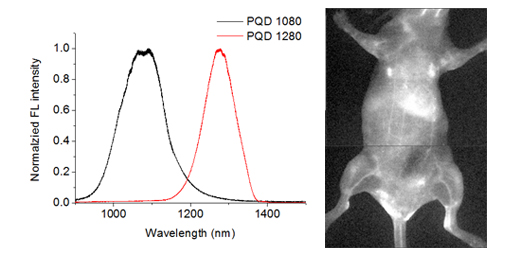 Figure 4. (left) PL spectra of two kinds of NIR-II emitting QDs (right) Wide-field NIR-II PL image of intravital mouse model administrated with NIR-II QDs by intravenous injection
Figure 4. (left) PL spectra of two kinds of NIR-II emitting QDs (right) Wide-field NIR-II PL image of intravital mouse model administrated with NIR-II QDs by intravenous injection
We report an enzyme-activatable NIR-II probe that exhibits fluorescence (FL) upon matrix metalloprotease activity in tumor microenvironment. Bright and stable PbS/CdS/ZnS core/shell/shell QDs were synthesized as a model NIR-II fluorophore, and activatable modulators were attached to exploit photoexcited electron transfer (PET) quenching. The quasi type-II QD band alignment allowed rapid and effective FL modulations with a compact surface ligand modulator that contains methylene blue PET quencher. The modulator was optimized to afford full enzyme accessibility and high activation signal surge when the enzyme was active. Using a colon cancer mouse model, the probe demonstrated selective FL activation at tumor sites with tripled signal strength in 10 min (Fig. 5).
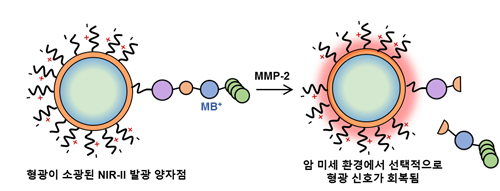 Figure 5. Displacement of the FL quencher, methylene blue, from a QD by the enzymatic cleavage of MMP.
Figure 5. Displacement of the FL quencher, methylene blue, from a QD by the enzymatic cleavage of MMP.
5. Sensing molecule by using QDs
Amphiphilic polyethyleneimine derivatives (amPEIs) were synthesized and used to encapsulate dozens of QDs. The QD-amPEI composite was ~100 nm in hydrodynamic diameter and had an outer surface with a slight positive charge that is well suited for cellular internalization. The outer positive surface was further exploited for gene delivery and targeting. Co-delivery of QDs and GFP-silencing RNAs was demonstrated by attaching siRNAs to the outer surface; the process achieved transfection efficiency that was an order of magnitude higher than conventional gene transfections. Hyaluronic acids were tethered onto the QD-amPEI for cell-specific targeted labeling, which achieved a specific-to-nonspecific signal ratio > 100. The inside hydrophobic compartment was further applied to cohost oxygen-sensing phosphorescence Ru dyes along with QDs. The ratiometric PL signals of the QD-Ru-amPEI oxygen probe show accurate and reversible oxygen-sensing capability and were successfully applied to cellular and spheroid models (Fig. 6).
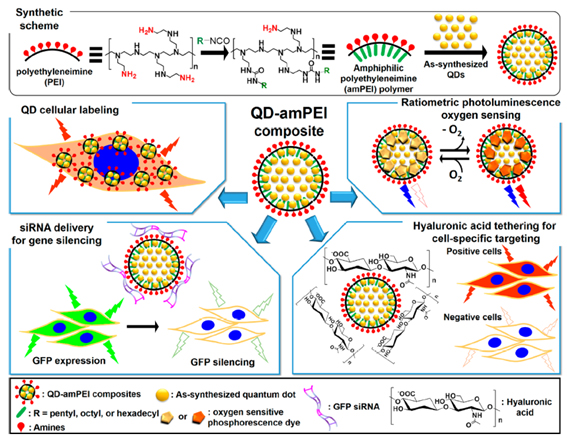 Figure 6. Schematic representations for the synthesis of amphiphilic polyethyleneimine derivatized polymer (amPEI) and the encapsulation of dozens of QDs in amPEI for QD-amPEI composite preparation (top), and demonstrated QD-amPEI platform applications: QD cellular labeling, siRNA delivery for GFP gene silencing, hyaluronic acid tethering for cell-specific targeting, and ratiometric PL oxygen sensing (from upper left box to counterclockwise order)
Figure 6. Schematic representations for the synthesis of amphiphilic polyethyleneimine derivatized polymer (amPEI) and the encapsulation of dozens of QDs in amPEI for QD-amPEI composite preparation (top), and demonstrated QD-amPEI platform applications: QD cellular labeling, siRNA delivery for GFP gene silencing, hyaluronic acid tethering for cell-specific targeting, and ratiometric PL oxygen sensing (from upper left box to counterclockwise order) ( ACS Nano , 2015, 9, 6511-6521)