RESEARCH
Nanoplasmonics
1. Properties of Surface Plasmon Resonance
Conductive metals contain large numbers of free electrons. They are not strongly bound to individual atoms, so the movements of the electrons can be easily controlled by applying external stimuli. Due to this behavior of free electrons, metals can display SPR when their sizes are reduced to the nanoscale. SPR gives unique optical properties to metal NPs. Especially, NPs made of noble metals such as gold and silver can interact strongly with visible light, and as a result, show intense absorptions and scatterings with absorption cross sections that are orders of magnitude larger than those of typical organic dyes. These NPs also resist oxidation and photo-bleaching. Therefore, noble-metal NPs can be highly potent optical probes, which can replace conventional organic dyes. The applications of noble metal NPs can be very diverse because plasmon resonance properties can be tailored by choosing metals with appropriate dielectric properties, and by selecting the size, shape, local environment of nanoscale metallic structures. Our goal is to design and synthesize various metallic nanostructures, and to reveal their optical properties by using spectroscopic and imaging tools. Finally we will apply the nanostructures, mainly in biological areas (Fig. 2).
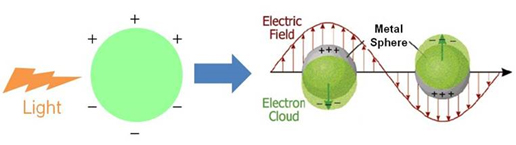 Figure 1. Illustration of Surface Plasmon Resonance (J. Phys. Chem. B, 2003, 107, 668-677)
Figure 1. Illustration of Surface Plasmon Resonance (J. Phys. Chem. B, 2003, 107, 668-677)
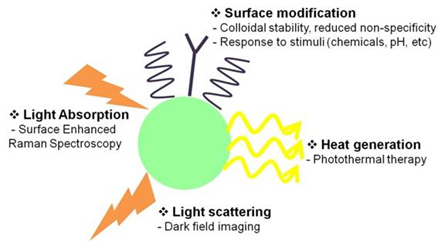 Figure 2. Biological applications of metal NP
Figure 2. Biological applications of metal NP
2. Study of thermotherapy for tumor cells
Photothermal therapy (Fig. 3) is a cancer treatment method in which photon energies are converted to thermal energy to kill cells. Cancer cells are generally more susceptible to heat than normal cells, so a selective temperature increase in cancer cells may maximize the therapeutic effect. Metal NPs can be highly potent photothermal therapeutic agents, due to their strong light absorption and efficient heat conversion. They can provide sufficient thermal energy to kill cancer cells. By controlling thermal energies produced by the photothermal effect of metal NPs, selectivity in cancer therapy can be achieved. Near-infrared (NIR) light penetrates deeply into live tissues because it is absorbed or scattered relatively weakly by biomolecules such as hemoglobin and water, so NIR is widely used for photothermal therapy. The photothermal therapeutic selectivity of pH-selective “Smart” gold NPs that absorb NIR light only in cancer cells and tissues can be further improved because of the unique environment of hypoxic tumor tissues and high frequency of exposure to the low-pH environment in cancer cells. Additionally, light-heat treatment that uses metal NPs can have synergistic effects with anti-cancer drugs. Aggregates of gold NPs can be achieved in many ways. As another example, synthesis of a complex of DNA hydrogel-NPs that releases anti-cancer drugs concurrently with aggregation of NPs, may lead to a synergistic therapeutic effect
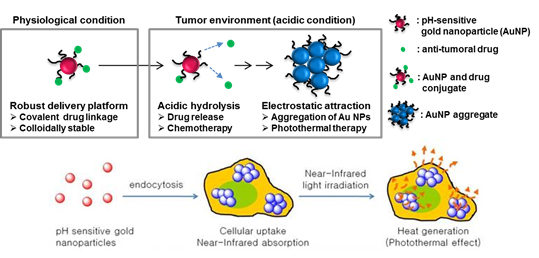 Figure 3. Application scheme of gold NPs in photothermal cancer therapy. (ACS Nano, 2013, 7, 3388-3402)
Figure 3. Application scheme of gold NPs in photothermal cancer therapy. (ACS Nano, 2013, 7, 3388-3402)
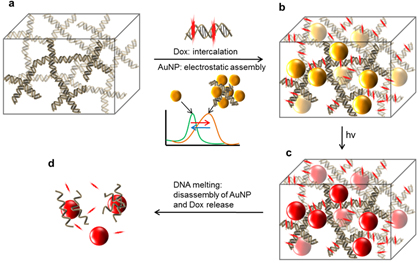 Figure 4. Photothermal therapy and chemotherapy using DNA hydrogel-gold NP complex that can absorb near-infrared light and anti-cancer drugs by DNA melting. (J. Chem. Mater. B, 2015, 3, 1537-1543)
Figure 4. Photothermal therapy and chemotherapy using DNA hydrogel-gold NP complex that can absorb near-infrared light and anti-cancer drugs by DNA melting. (J. Chem. Mater. B, 2015, 3, 1537-1543)
3. Dark-field microscopy
Dark-field microscopy (DFM) collects scattered light from a sample specimen to show its scattering properties, whereas in typical transmittance-mode microscopy, transmitted light is used to form images. Metal NPs can display distinct images under DFM because NPs strongly scatter incident light that is in resonance with their own SPR. Furthermore, even materials with similar refractive indexes can be distinguished using DFM because scattering is generally very weak and is independent of the refractive index. DFM enables characterization of the SPR property of even single metal NPs, and imaging of biological materials using metal NPs as contrast agents (Fig. 5).
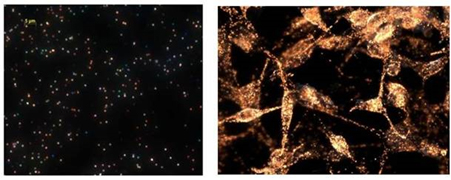 Figure 5. Dark-field microscope image of silver nanoparticles (left) and gold nanoparticles within cancer cells (right).
Figure 5. Dark-field microscope image of silver nanoparticles (left) and gold nanoparticles within cancer cells (right). (J. Am. Chem. Soc., 2009, 131, 13639-13645)
4. Photoacoustic imaging using ‘smart’ gold NPs.
Photoacoustic (PA) imaging (PAI) is an emerging hybrid imaging mode that combines the concepts of ultrasound and optical imaging. PAI is a nonionizing biomedical imaging method that can provide molecular functional information such as hemoglobin oxygen concentration, blood flow, and morphology of living tissues. PAI is suitable for various biomedical studies including monitoring physiological reactions of tumors. In PAI, the contrast of an image depends on the optical absorption property of the target objects. An exogenous contrast agent that absorbs strongly in the near-infrared (NIR) region can sharpen the contrast of the PA image due to the deep tissue penetration. ‘Smart’ gold NPs (SANs) can rapidly form aggregates in mild acidic environments, which general occur within tumors. As a result of this aggregation, their absorption spectrum red-shifts to red and NIR wavelengths. PA amplitude increases in mild acidic conditions, but does not change much at physiological pH. By exploiting large accumulation of NPs in tumors, and high PA conversion efficiency, a the signal has been amplified significantly in cell-level and small-animal experiments (Fig. 6). SANs may provide a new platform for PA-guided drug delivery that identifies delivery targets and promptly administers drugs in real time.
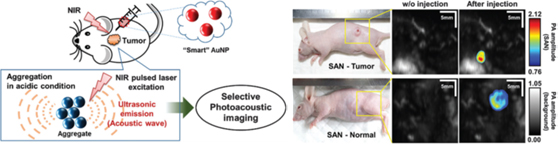 Figure 6. “Smart" gold nanoparticles aggregate rapidly in tumor microenvironment, and this aggregation results in a shift in the absorption peak to the near-infrared region. The cancer-selective NIR absorption is exploited for PA imaging between tumor and normal tissues. (Chem. Commun., 2016, 52, 8287-8290).
Figure 6. “Smart" gold nanoparticles aggregate rapidly in tumor microenvironment, and this aggregation results in a shift in the absorption peak to the near-infrared region. The cancer-selective NIR absorption is exploited for PA imaging between tumor and normal tissues. (Chem. Commun., 2016, 52, 8287-8290).